 2. Addition Reactions Initiated by Electrophilic Halogen 2. Addition Reactions Initiated by Electrophilic Halogen
 The halogens chlorine and bromine add rapidly to a wide variety of alkenes without inducing the kinds of structural rearrangements noted for strong acids (first example below). The stereoselectivity of these additions is strongly anti, as shown in many of the following examples. The halogens chlorine and bromine add rapidly to a wide variety of alkenes without inducing the kinds of structural rearrangements noted for strong acids (first example below). The stereoselectivity of these additions is strongly anti, as shown in many of the following examples.

 An important principle should be restated at this time. The alkenes shown here are all achiral, but the addition products have chiral centers, and in many cases may exist as enantiomeric stereoisomers. An important principle should be restated at this time. The alkenes shown here are all achiral, but the addition products have chiral centers, and in many cases may exist as enantiomeric stereoisomers.
 In the absence of chiral catalysts or reagents, reactions of this kind will always give racemic mixtures if the products are enantiomeric. On the other hand, if two chiral centers are formed in the addition the reaction will be diastereomer selective. This is clearly shown by the addition of bromine to the isomeric 2-butenes. Anti-addition to cis-2-butene gives the racemic product, whereas anti-addition to the trans-isomer gives the meso-diastereomer. In the absence of chiral catalysts or reagents, reactions of this kind will always give racemic mixtures if the products are enantiomeric. On the other hand, if two chiral centers are formed in the addition the reaction will be diastereomer selective. This is clearly shown by the addition of bromine to the isomeric 2-butenes. Anti-addition to cis-2-butene gives the racemic product, whereas anti-addition to the trans-isomer gives the meso-diastereomer.
 We can account both for the high stereoselectivity and the lack of rearrangement in these reactions by proposing a stabilizing interaction between the developing carbocation center and the electron rich halogen atom on the adjacent carbon. We can account both for the high stereoselectivity and the lack of rearrangement in these reactions by proposing a stabilizing interaction between the developing carbocation center and the electron rich halogen atom on the adjacent carbon.
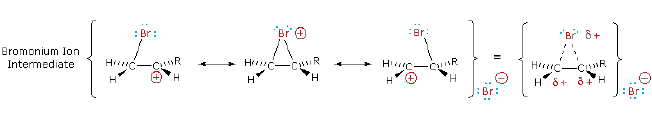
 This interaction, which is depicted for bromine in the following equation, delocalizes the positive charge on the intermediate and blocks halide ion attack from the syn-location. This interaction, which is depicted for bromine in the following equation, delocalizes the positive charge on the intermediate and blocks halide ion attack from the syn-location.

 The stabilization provided by this halogen-carbocation bonding makes rearrangement unlikely. In a few cases three-membered cyclic halonium cations have been isolated and identified as true intermediates. The stabilization provided by this halogen-carbocation bonding makes rearrangement unlikely. In a few cases three-membered cyclic halonium cations have been isolated and identified as true intermediates.
 A resonance description of such a bromonium ion intermediate is shown below. The positive charge is delocalized over all the atoms of the ring, but should be concentrated at the more substituted carbon (carbocation stability), and this is the site to which the nucleophile will bond. A resonance description of such a bromonium ion intermediate is shown below. The positive charge is delocalized over all the atoms of the ring, but should be concentrated at the more substituted carbon (carbocation stability), and this is the site to which the nucleophile will bond.
|